Research Study
Sona’s Cancer Therapy Triggers Abscopal Effect, Eliminating Distant Tumors In Preclinical Melanoma Study
A detailed biomarker analysis of the recently reported pre-clinical melanoma study conducted at Dalhousie University (the “Study”) indicates that, beyond shrinking tumors on its own, Sona’s Targeted Hyperthermia Therapy (“THT”) also stimulates the innate immune system to target and eliminate untreated (contralateral) tumors when combined with a standard immunotherapeutic drug, IL-2. (See figure #1, below.) On the basis of efficacy data achieved in two different murine cancer models (triple negative breast cancer and malignant melanoma), the Company is now proceeding with safety and biocompatibility testing that will be required by regulating agencies to enter into human studies.
Sona’s Chief Medical Officer and the Study’s principal investigator, Dr. Carman Giacomantonio, commented, “With two separate murine cancer models completed, we now believe it is clear that Sona’s THT therapy causes cancer specific proteins (cancer antigens) to become visible to the immune system. This in turn causes novel, innate immune responses ultimately enabling development of cancer-specific immunity. This is essentially the goal of all current immunotherapy research and treatment strategies. When combined with a standard of care immunotherapeutic drug (IL-2), the resultant immunity in our models was strong enough to generate an immune response in remote (contralateral) tumors. Finally, we were excited to observe that the immunity generated by Sona’s gold nanorod-based THT therapy in our preclinical models is lasting. In other words, we observed a “vaccine effect” whereby we were unable to immediately grow new tumors in mice whose primary tumors had responded to Sona’s gold nanorod THT therapy combined with standard immunotherapy. This is the proof of concept we were looking for.”
Following treatment with Sona’s THT for melanoma in mice, an analysis of key metrics of immune memory showed that remote tumors could not be established in mice with primary tumors previously treated with Sona’s gold nanorod-based THT therapy when combined with standard (IL-2) immunotherapy. Furthermore, an examination of the upregulation of inflammatory gene expression for 17 genes for both Sona’s 4T1 (triple negative breast cancer) and B16 (melanoma) models showed a strong pattern of expression enhancement, a further indication of the success Sona’s THT therapy had in effectuating a fundamental change to the innate immune system. (See figure #2, below.)
Sona CEO, David Regan, commented, “Seeing the gene expression data which supports the permanency of the new immunity achieved and the fact that new cancer tumors did not take in the treated mice in this preclinical study gives hope that Sona’s therapy could be used with immunotherapeutic drugs to treat cancer effectively and reduce the likelihood of recurrences. Given our success in demonstrating this concept in two different murine cancer models, we are now moving forward aggressively with the study program required by regulators that would allow us to progress this novel therapy into the clinic for a first-in-human trial [which we aim to achieve in 2025]. Our initial indication will target the thousands of people who suffer from late-stage, unresectable melanoma for which no other therapy has worked.”
The results discussed in this release are preliminary and have not been subject to peer review. Upon completion, the Company expects that the full Study will be submitted for peer review and scientific journal publication.
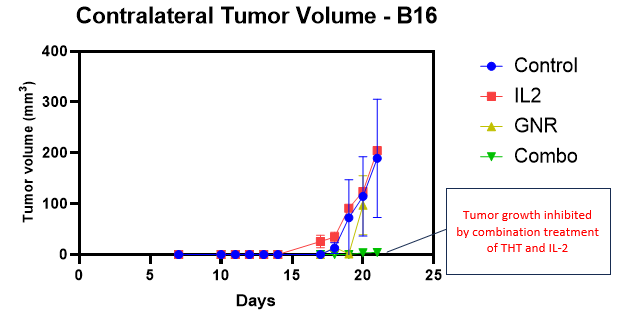
Figure #1: Sona’s THT Therapy Combined with IL-2 Inhibits Growth of Distant Tumors
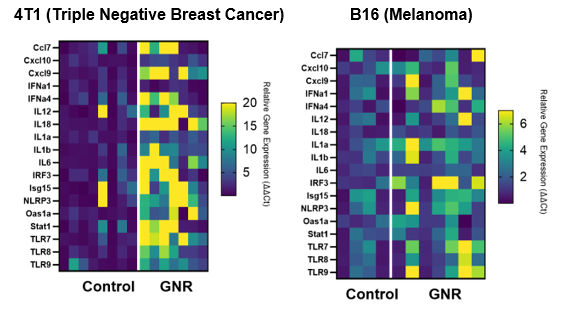
Figure #2: Gene Expression Heat Maps
Read More About Targeted Hyperthermia Therapy
- Preclinical Small Animal Study Success
- First THT Application: Colorectal Cancer Tumors