Leveraging GNR Gold Nanorod
Sona’s GNR technology in Siva Therapeutics’

Uniquely biocompatible

Unlocking of in-vivo medical applications potential


Pioneered by
Len Pagliaro, PhD
- 24 years of experience
- Successful commercialization of biotechnology products, services, and licensing
- Completed prestigious assessment program at the US Nanotechnology Characterization Laboratory (Established by the FDA and the National Cancer Institute)
Sona GNRs in targeted hyperthermia may create the opportunity to treat cancer without doing significant harm to healthy cells
Current Cancer Treatments are risky, expensive and can do harm
- Chemotherapy and radiotherapy are non-selective in their destruction of cells;
- Advanced therapies are expensive;
- Surgery is risky.
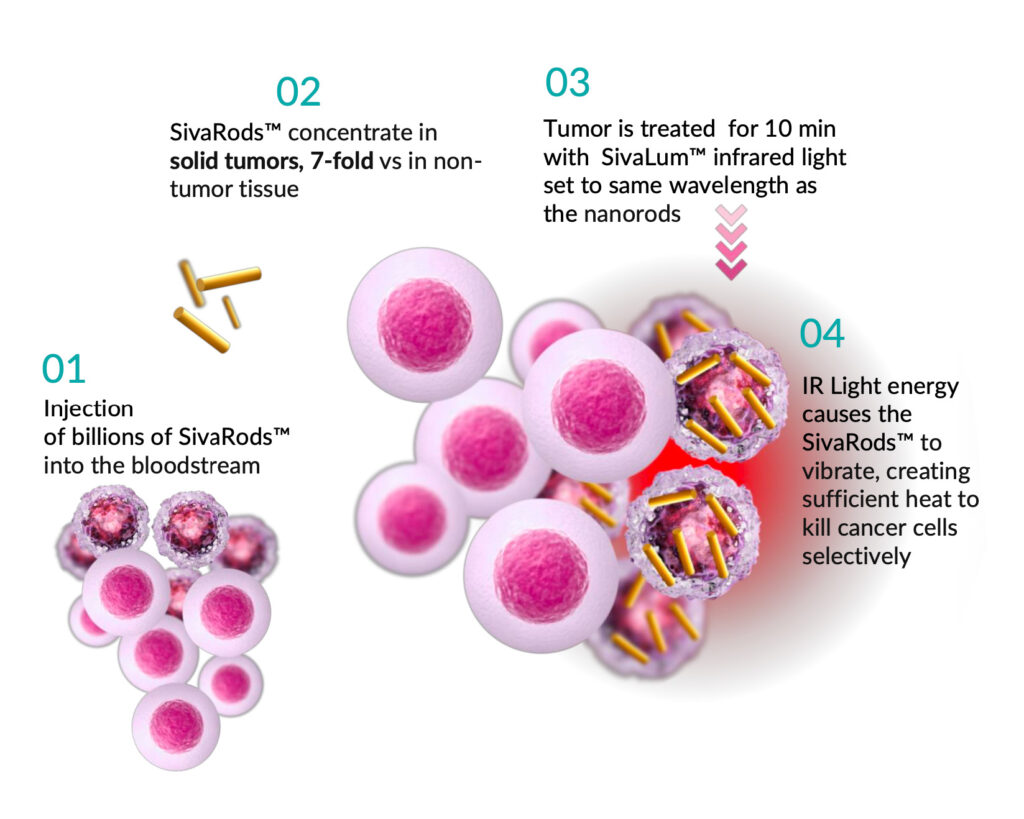
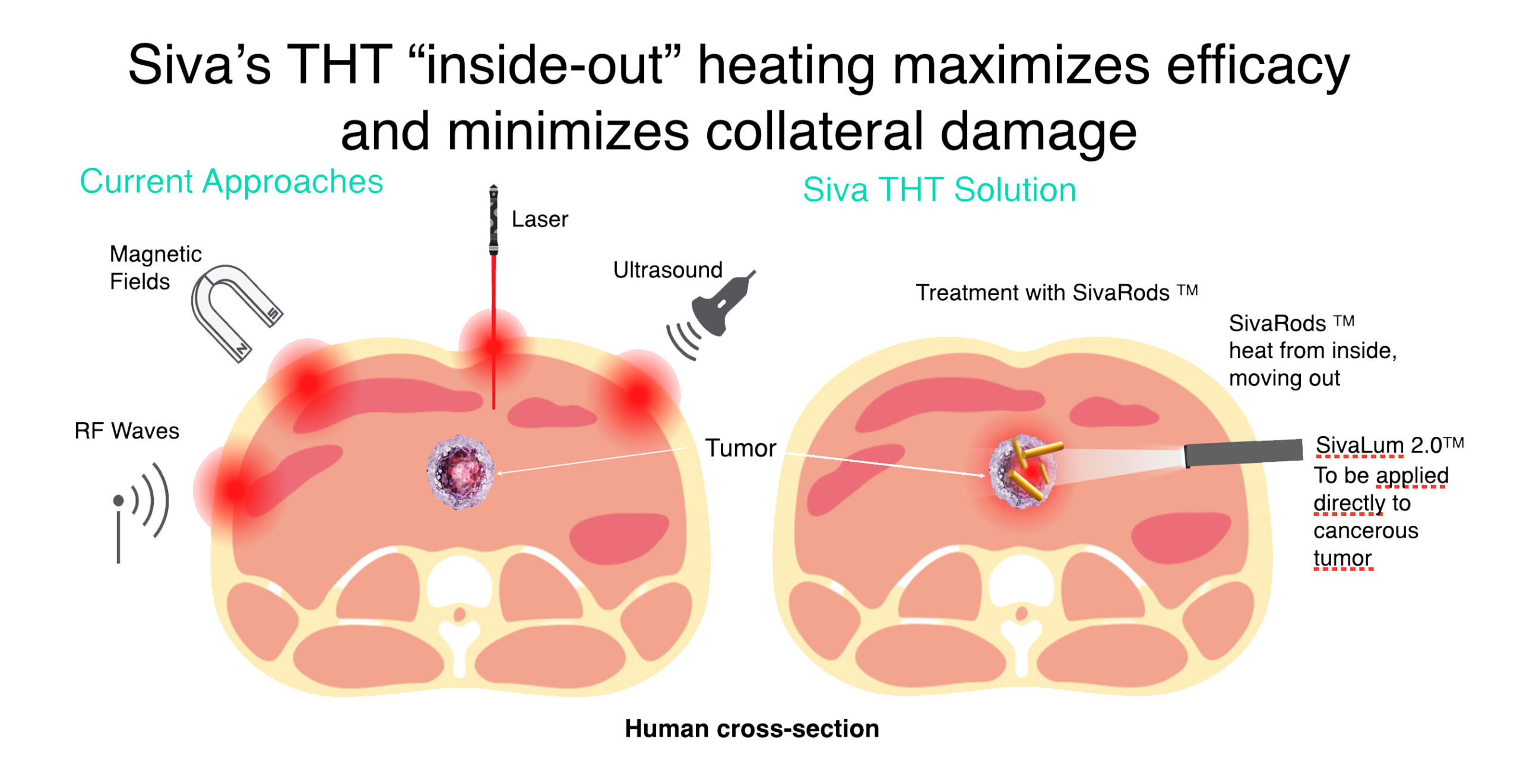
How does THT work?
- Targets heat directly to the tumor;
- Uses IV injection of GNRs to heat the tumor from the inside;
- Achieves hyperthermia instead of ablation destroying cancerous cells selectively;
- Sona GNRs are inert and do not use toxic CTAB.
Targeted Hyperthermia Therapy™ (THT)
Medical device with two components:
- Gold nanorods for injection
- Infrared light source
Heating tumors does the following:
- Stimulates immune system
- Kills cancer cells
- Increases tumor perfusion
- Shrinks tumors
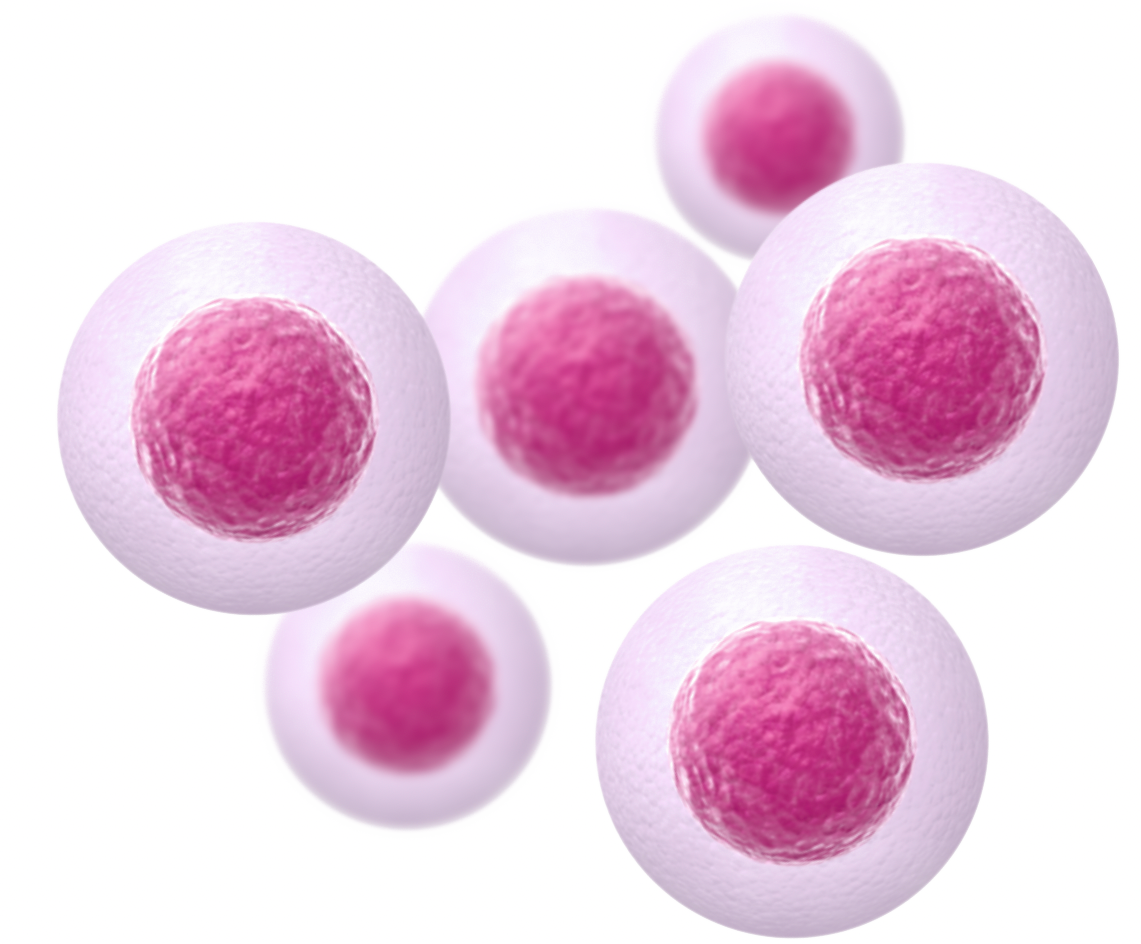
Healthy cells withstand heat stress, up to 52°C, typical with ablation therapies.
Not damaged by hyperthermia’s 44°C
THT destroys cancer cells while healthy cells can stay undamaged
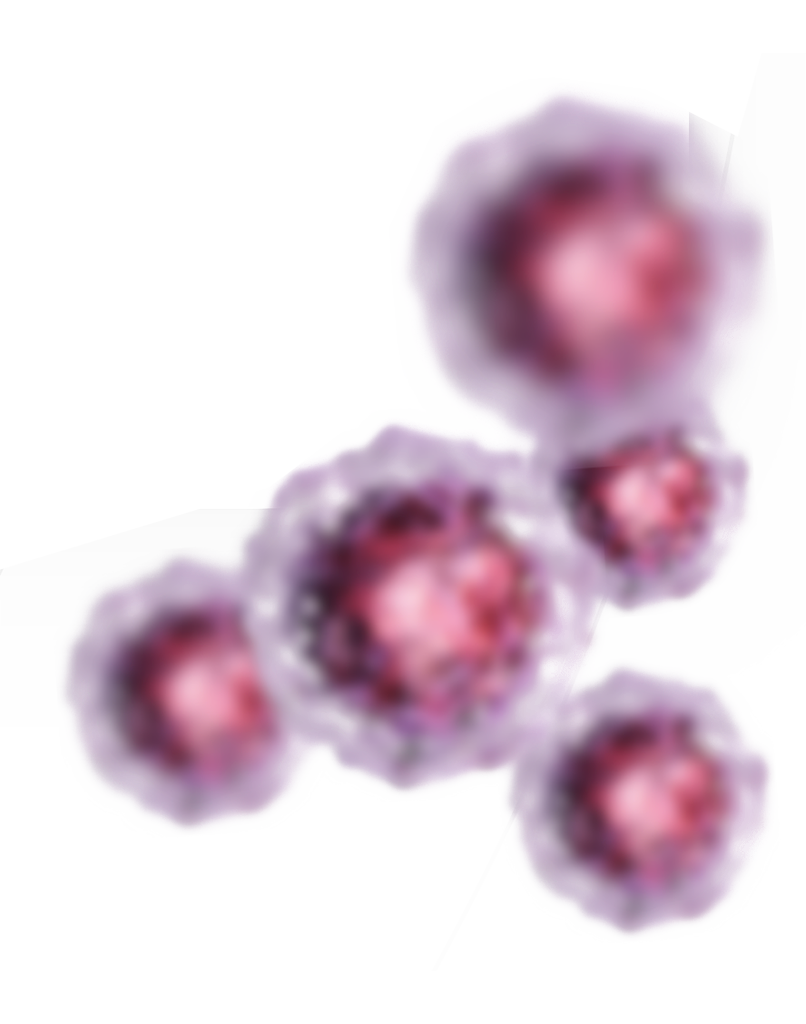
SivaRods™ heat to 44°C
Selectively kills cancer cells
Works from the inside of the tumor out
Heat shock protein (HSP) synthesis used

Cancer cells are more sensitive to heat
Siva’s Unique Differentiator:
Selective ‘Hyperthermia’ Minimizes Collateral Damage
- THT photothermal cancer therapy using GNRs will address current treatment issues.
- Siva's THT "inside-out" heating maximizes efficacy and minimizes collateral damage.

Hyperthermia
(44°C, cooler than blue rare!)
Blue Rare (115°F) i.e. 46°C
Hyperthermia doesn’t harm normal cells

Ablation
(> 55°C)
Medium (134°F) i.e. 57°C

Ablation therapies ‘cook’ proximate cells
- Siva has had major success in reducing tumors in small animal studies.
- Eliminated tumors in mice in 4 weeks
- Validated by Nanotechnology Characterization Laboratory study and report.
THT photothermal cancer therapy using GNRs will address current treatment issues
Key Issue
Using gold ‘in vivo’ is understood to be safe
Long-term effects of GNRs treated with toxic CTAB are unknown.
Sona GNR Advantage
Sona’s proprietary CTAB-free GNRs have shown no toxicity
Confirmed by third party and in-house testing

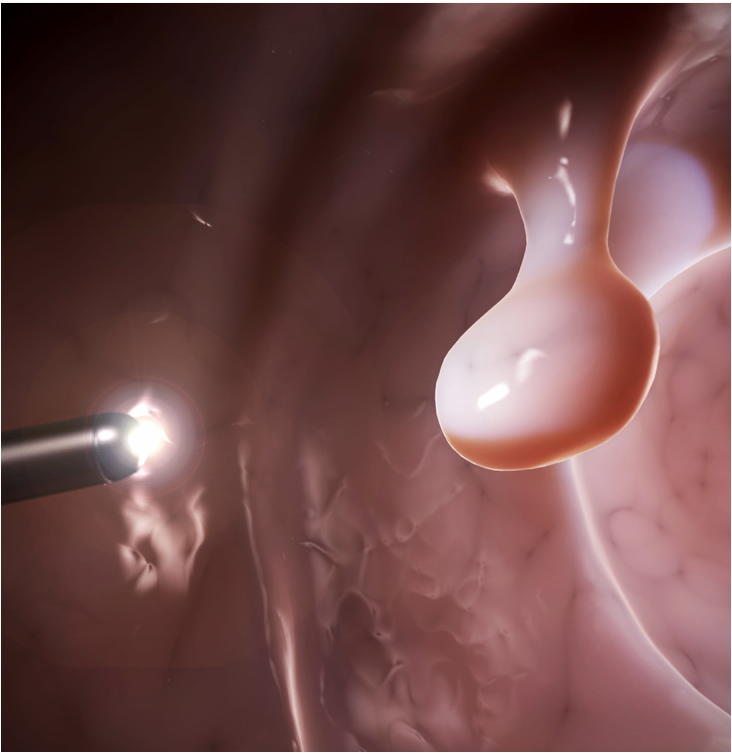



First THT Application: Colorectal Cancer Tumors
Why is THT uniquely suited for colorectal cancer treatment?
- Alternatives diminish quality of life
- Significant market
- Outpatient procedure within current workflow
- Effective for solid tumors
- Early detection is possible
- THT can be integrated with “watch and wait” approach
- Low metastatic index
Treatment Benefits:
- Minimally invasive
- Targeted treatment
- Enhances success of other cancer therapies
- Easy to use
- Affordable
Read More About Targeted Hyperthermia
- Preclinical Small Animal Study Success
- First THT Application: Colorectal Cancer Tumors
Road to Commercialization
Potential future clinical studies to provide multiple valuation catalysts.
Additionnal Publications

Sona Announces Canadian Melanoma Clinical ‘Pilot’ Study Ethics Approval
Sona Nanotech Inc. (CSE: SONA) (OTCQB: SNANF) (the “Company”, “Sona”), an oncology-focused life sciences company developing innovative therapies based on its uniquely biocompatible gold nanorod technology, has received Nova Scotia Health Research Ethics Board (“REB”) approval to conduct its proposed pilot human clinical trial study (the “Pilot Study”) of its Targeted Hyperthermia Therapy (“THT”) cancer treatment with late-stage melanoma patients. Sona’s THT Pilot Study is being planned as a multi-centre clinical trial for 30-40 patients and is subject to, amongst other things, securing medical device Investigational Testing Authorization (“ITA”) from Health Canada.