Sona's THT Leverage Its Uniquely Biocompatible Gold Nanorods
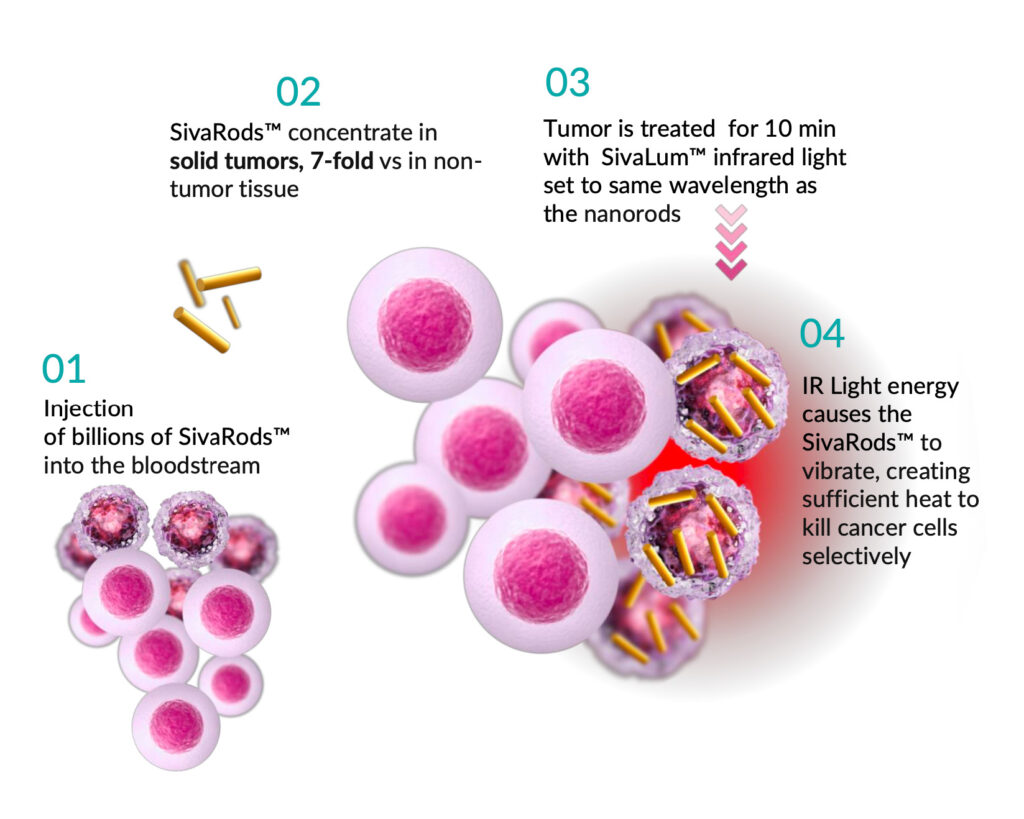
Sona Nanotech’s Targeted Hyperthermia Therapy (THT) is a next-generation cancer treatment that uses biocompatible gold nanorods (GNRs) to deliver therapeutic heat directly to solid tumors. By harnessing the unique properties of these nanorods, THT offers a minimally invasive, targeted approach designed to destroy cancer cells while sparing healthy tissue. This innovative therapy aims to address the limitations and side effects of traditional cancer treatments, offering new hope for patients with resistant or advanced cancers

Uniquely biocompatible

Unlocking the potential of in-vivo medical applications

How does THT work?
Sona’s Patented Gold Nanorods Heat Tumors Gently From The Inside, when exposed neon infrared light of an appropriate wave length. Causing Selective Apoptotic Cell Death* Which Reveals Neoantigens
- Precise Delivery: Gold nanorods are injected into the tumor.
- Activation by Infrared Light: Once in place, the nanorods are activated by near-infrared (NIR) light, which penetrates the skin and is absorbed by the GNRs.
- Localized Heating: The absorbed light is converted to heat (42–48°C), destroying cancer cells from within while leaving surrounding healthy tissue unharmed.
- Immune Engagement: Destroying cancer cells but also exposes tumor neoantigens, which can stimulate the patient’s immune system to recognize and attack cancer cells, including those previously hidden from immune detection
*Apoptotic Cell Death:
When a cell actively self-destructs in a controlled manner. In so doing, the dying cells become more “visible” to immune cells due to the altered antigen presentation which can cause the immune system to engage and attack the cancer
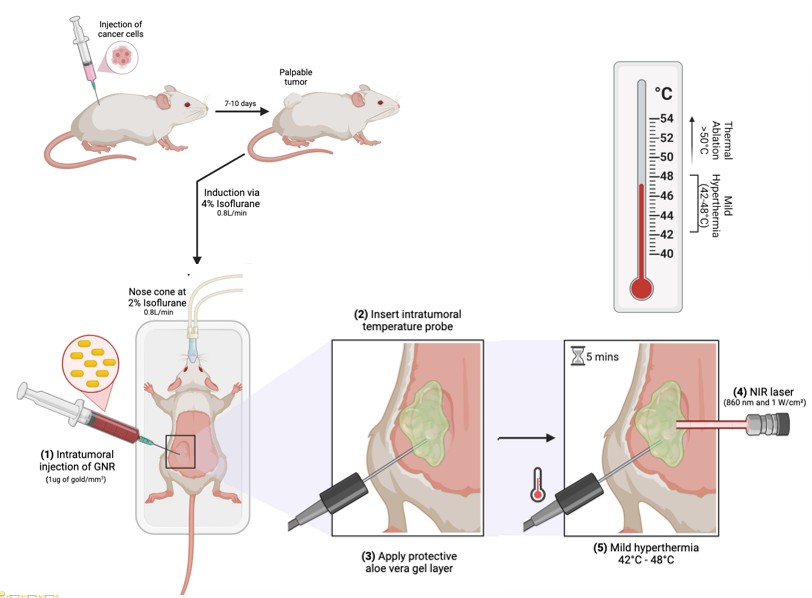
Sona’s THT Involves Two Injections Of GNRs Followed By ~5 Minutes Of Sona’s Near-infrared Laser Energy Which GNRs Convert To Heat
Clinical Progress & Recent Milestones
First-in-Human Dosing
In July 2025, Sona Nanotech achieved a significant milestone by dosing the first patient in an early feasibility clinical trial for advanced melanoma. This trial is designed to assess the safety, tolerability, and preliminary efficacy of THT in patients who have not responded to standard immunotherapies.
Study Design
The study involves two THT treatments given one week apart, with key endpoints including tumor growth inhibition and immune system activation.
Preclinical Success
Earlier studies demonstrated that THT can convert immune-resistant “cold” tumors into “hot” tumors, making them more susceptible to immunotherapy and resulting in strong anti-tumor responses.
Ongoing Evaluation
Initial clinical results are expected in summer 2025, with final outcomes anticipated in the fall. These insights will guide further clinical development and potential expansion to other cancer types.
Next Gen Laser
Sona’s Next Generation Laser is a custom-engineered, near-infrared light device designed specifically for use with Sona’s gold nanorod (GNR) technology in Targeted Hyperthermia Therapy (THT). This device has undergone extensive validation and verification to ensure it reliably delivers consistent energy output with robust safety features, meeting the stringent requirements of clinical trials and regulatory bodies
- Optimized wavelength (860 nm): Penetrates up to one inch into tissue, allowing precise targeting of tumors from the surface or within body cavities
- Ergonomic, pen-like handpiece: Designed for clinician comfort and flexible, accurate tumor targeting during treatment
- Consistent, reliable energy delivery: Rigorously validated for clinical use, with built-in safety features to ensure dependable performance
- Non-invasive approach: Enables access to tumors without the need for surgical procedures, supporting minimally invasive cancer therapy

Unlike surgical lasers that cut or ablate tissue, Sona’s laser gently transmits non-thermal near-infrared light, which is absorbed by the injected gold nanorods. These nanorods then convert the light into heat, selectively destroying cancer cells from the inside out while sparing healthy tissue. This approach not only treats tumors directly but also has the potential to stimulate the immune system, supporting Sona’s vision for innovative, minimally invasive cancer therapies

Pioneered by
Len Pagliaro, PhD
- 24 years of experience
- Successful commercialization of biotechnology products, services, and licensing
- Completed prestigious assessment program at the US Nanotechnology Characterization Laboratory (Established by the FDA and the National Cancer Institute)
Sona GNRs in targeted hyperthermia may create the opportunity to treat cancer without doing significant harm to healthy cells
Current Cancer Treatments are risky, expensive and can do harm
- Chemotherapy and radiotherapy are non-selective in their destruction of cells;
- Advanced therapies are expensive;
- Surgery is risky.
Advantages & Future Potential
Minimally Invasive
THT targets tumors with precision, reducing collateral damage and side effects compared to chemotherapy, radiation, or surgery.
Immune System Activation:
By exposing tumor antigens, THT has the potential to enhance the effectiveness of immunotherapies and treat tumors that are otherwise resistant.
Versatile Platform
The technology is being explored for multiple cancer types, with future studies planned to expand its application.
Safety & Biocompatibility
Sona’s gold nanorods are CTAB-free, eliminating toxicity risks associated with other nanoparticle technologies and supporting safe use in humans
Clinical Integration
THT is designed to be a valuable adjunct to existing cancer treatments, potentially improving patient outcomes and quality of life
How does THT work?

- Targets heat directly to the tumor;
- Uses IV injection of GNRs to heat the tumor from the inside;
- Achieves hyperthermia instead of ablation destroying cancerous cells selectively;
- Sona GNRs are inert and do not use toxic CTAB.
Targeted Hyperthermia Therapy™ (THT)
Medical device with two components:
- Gold nanorods for injection
- Infrared light source
Heating tumors does the following:
- Stimulates immune system
- Kills cancer cells
- Increases tumor perfusion
- Shrinks tumors
Healthy cells withstand heat stress, up to 52°C, typical with ablation therapies.
Not damaged by hyperthermia’s 44°C
THT destroys cancer cells while healthy cells can stay undamaged
SivaRods™ heat to 44°C
Selectively kills cancer cells
Works from the inside of the tumor out
Heat shock protein (HSP) synthesis used

Cancer cells are more sensitive to heat
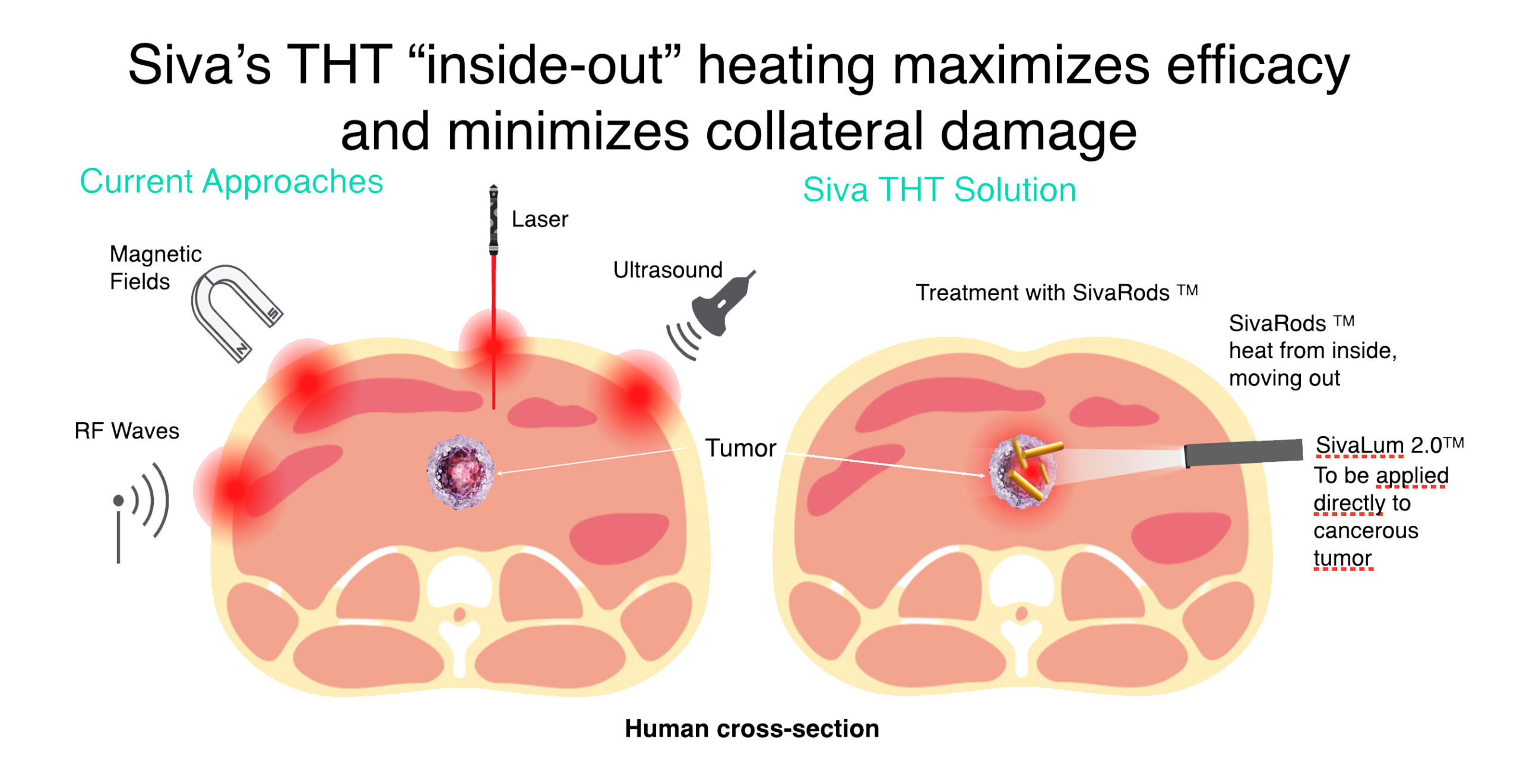
Siva’s Unique Differentiator:
Selective ‘Hyperthermia’ Minimizes Collateral Damage
- THT photothermal cancer therapy using GNRs will address current treatment issues.
- Siva's THT "inside-out" heating maximizes efficacy and minimizes collateral damage.

Hyperthermia
Blue Rare (115°F) i.e. 46°C
Hyperthermia doesn’t harm normal cells

Ablation
Medium (134°F) i.e. 57°C

Ablation therapies ‘cook’ proximate cells
- Siva has had major success in reducing tumors in small animal studies.
- Eliminated tumors in mice in 4 weeks
- Validated by Nanotechnology Characterization Laboratory study and report.
THT photothermal cancer therapy using GNRs will address current treatment issues
Key Issue
Using gold ‘in vivo’ is understood to be safe
Long-term effects of GNRs treated with toxic CTAB are unknown.
Sona GNR Advantage
Sona’s proprietary CTAB-free GNRs have shown no toxicity
Confirmed by third party and in-house testing

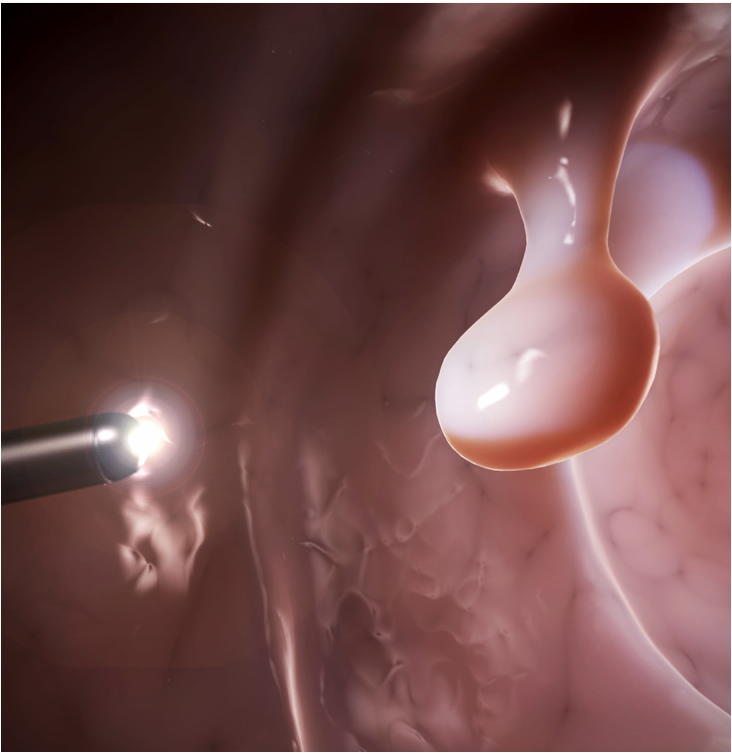



First THT Application: Colorectal Cancer Tumors
Why is THT uniquely suited for colorectal cancer treatment?
- Alternatives diminish quality of life
- Significant market
- Outpatient procedure within current workflow
- Effective for solid tumors
- Early detection is possible
- THT can be integrated with “watch and wait” approach
- Low metastatic index
Treatment Benefits:
- Minimally invasive
- Targeted treatment
- Enhances success of other cancer therapies
- Easy to use
- Affordable
Read More About Targeted Hyperthermia
- First Patient Dosed in Early Feasibility Study Trial of Its Cancer Therapy
Road to Commercialization
Potential future clinical studies to provide multiple valuation catalysts.
Additionnal Publications